Thank You - Intrinsic Viscosity for Optimization of Protein Therapeutics
Application Note
Intrinsic Viscosity for Optimization of Protein Therapeutics
Key Words: intrinsic viscosity, injectability, protein screening, Bovine Serum Albumin, Concentration,
proteins
Goal: The following application note explains how intrinsic viscosity allows molecular properties to be determined from viscosity measurements.
Introduction - What is Intrinsic Viscosity?
Intrinsic viscosity measurements help to understand the protein-protein and protein-media interactions which affect efficacy, stability, and shelf life. It can also be utilized to estimate the viscosity of highly concentrated protein solutions which determines the injectability. This estimation of the viscosity in the concentrated regime, using small sample volumes, enable screening of proteins in early stage development.
Viscosity is the parameter that characterizes the resistance of a fluid to flow: a higher viscosity fluid provides higher resistance. When proteins are added to aqueous media, viscosity increases with concentration because more work (or stress) is required to move the larger and more viscous protein molecules than low viscosity media. The intrinsic viscosity is defined as the rate of the viscosity increase with concentration as shown below:
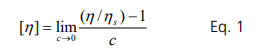
The rate of the viscosity increase is proportional to the hydrodynamic volume (Vh) of the molecule added to the media. Thus, the intrinsic viscosity, [η], is given by the Einstein-Simha equation for a spherically shaped object:
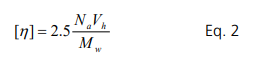
Na is the Avogadro constant, Vh is the hydrodynamic volume, and Mw is the molecular weight. Therefore, hydrodynamic volume, or radius, can be calculated from the intrinsic viscosity measurement. To measure the intrinsic viscosity, viscosities of protein solutions need to be measured at different dilute concentrations. The concentration regime for the valid intrinsic viscosity measurement is discussed in the last section. The hydrodynamic radius is correlated with interactions between proteins and media (pH, ionic strength, buffer type, etc.).
Viscosity Increase with Concentration
The three drawings below are a graphical representation of the protein population depending on concentration in the dilute regime.
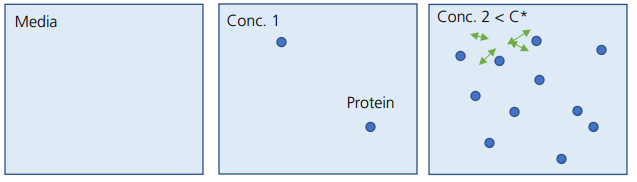
(Arrows indicate thermodynamic and hydrodynamic interactions between molecules when C is increased.)
At very low concentrations (Conc. 1), protein molecules are far apart from each other and thus interaction between molecules is negligible. With no interaction between molecules, viscosity increases proportionally with concentration. As the concentration is increased, as seen in the third drawing (Conc. 2), protein molecules start interacting with each other.
The interaction between molecules is both thermodynamic and hydrodynamic in nature. The thermodynamic component is attributed to protein-protein interaction consisting of both a short-range attraction and long-range repulsion (SALR). Perturbation of the thermodynamic interactions among molecules by shearing induces increase of viscosity due to required additional work. Movement of a molecule induces movement of another molecule through a hydrodynamic interaction, which also increases the viscosity. The binary interaction between two neighboring molecules increases the viscosity in
proportion to C2 and thermodynamic as well as hydrodynamic interactions are included in the constant k in Eq. 3.
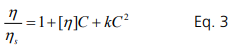
Note that higher order terms are not included. As the concentration is increased further, tertiary or higher order interactions become dominant. Therefore, Eq. 3 is only valid at low concentrations. The maximum valid concentration (C*) for application is discussed in the last section. The equation is rearranged to become the Huggins’s equation. It is important to note that [η]C scales with the volume fraction.
![]()
KH is the Huggins’s constant, ηs is the solvent viscosity and ηr is the relative viscosity. Alternatively, the Kraemer equation has been also used for the intrinsic viscosity measurement.
![]()
If the concentration is sufficiently small, then the following relationship should hold:
![]()
KH and KK provides the extent of thermodynamic and hydrodynamic interactions between molecules.
Measurement of Intrinsic Viscosity
In order to calculate the intrinsic viscosity, viscosities of solutions at low concentrations need to be measured. The data is
plotted so that it can be fit with the rearranged forms of Eq. 4 and 5 as shown below.
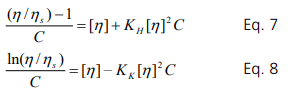
The quantities on the left-hand side of both equations are plotted against the concentration as shown in the graph below. The intercept on the ordinate yields the intrinsic viscosity and the slope of the curves provides the Huggins and Kraemer constants following the Eqs 7 and 8.
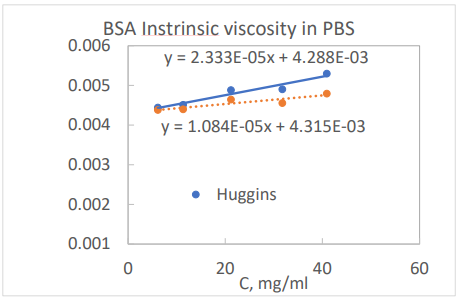
The graph below shows both plots for a bovine serum albumin (BSA) in PBS solution. As shown in the graph, both Huggins and Kraemer plots intercept at the intrinsic viscosity of 0.0043 ml/mg. From the slopes of the lines and the intrinsic viscosity, both dimensionless constants, KH and KK, can be calculated and are listed in the table below.
![]()
Importance of Intrinsic Viscosity & Huggins and Kraemer
The hydrodynamic radius can be calculated from the intrinsic viscosity. For a spherical particle, Eq. 2 is rearranged for the hydrodynamic radius, rh:
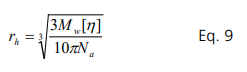
For the BSA in PBS system, rh is calculated to be 3.6 nm, which is close to the reported values measured by other techniques such as dynamic light scattering. The hydrodynamic radius is the size of the hydrated protein molecule. Changes of the hydrodynamic radius with types of media indicates the interaction between protein molecules and media. Compact and smaller molecular protein size is expected if the interaction between protein molecules and media favors stability. One can expect that protein molecules become larger with the presence of denaturant or as the temperature increases Huggins constant provides
the interaction between protein molecules.
Also, Kraemer’s constant provides insight about how fast the viscosity increases with concentration as in Eq. 6. While Eq. 6 is only valid at low concentrations, the Kraemer constant is proven experimentally to predict the viscosity of protein solutions even at high concentration up to 200 mg/ml with good accuracy. For those who are interested in learning the prediction, please contact us or look at the application note, “Prediction of viscosity of protein solution at 200 mg/mL from the accurate measurement of low concentration solutions”.
Concluding Remarks What is the Maximum Concentration for Intrinsic Viscosity Measurements?
An order of magnitude estimation of the intrinsic viscosity can be estimated from the molecular weight of protein using Eq. 2. Then the maximum concentration can be estimated from Eq. 3 with the constraint that viscosity increase over solvent, [η]C, is less than 20% or 0.2. Please note that [η]C scales with the volume fraction of the protein molecules in the media. After the intrinsic viscosity measurement is obtained, one can do a sanity check for the maximum concentration by calculating 0.2/[η]. For the BSA solution in PBS, the maximum concentration is calculated to be 0.2/0.0043 = 46 mg/mL. The maximum concentration suggests that concentration ranges were properly selected for the intrinsic viscosity measurement shown in the graph.
Concluding Remarks
Intrinsic viscosity allows molecular properties to be determined from viscosity measurement. The hydrodynamic radius, corresponding to stability and interaction between protein and media, can be determined from the resulting intrinsic viscosity. Additionally, protein-protein interaction as well as prediction of viscosity at higher concentrations can be determined from KH and Kk.
To summarize the applications of the intrinsic viscosity measurement:
• Hydrodynamic radius
• Interaction between protein and media with pH, ionic strength, buffer, etc.
• Protein-protein interaction through KH and KK
• Prediction of viscosity of antibody solution at 200 mg/mL from the intrinsic viscosity measurement
• Protein stability and aggregation
• Diffusivity calculated from Stokes-Einstein relation and hydrodynamic radius
If this note is helpful, please let us know! If you have questions or need more information about this product or other applications, please contact us:
Main Office — 1 925 866 3801
Sales – Sales@RheoSense.com
Information — info@RheoSense.com
.png?width=200&height=58&name=RheoSense%20Logo%20(REGISTERED).png)