Viscosity Temperature-Dependence of Battery Solutions
Application Note Download
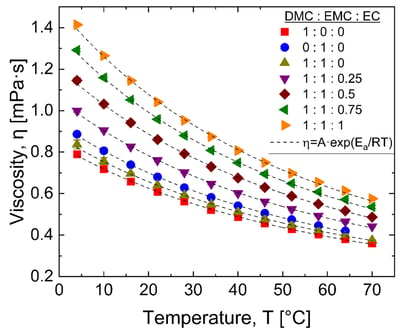
Lithium-ion battery electrolyte solutions are typically composed of a binary mixture of cyclic carbonates, e.g., ethylene carbonate (EC), and linear carbonates, e.g., dimethyl carbonate (DMC) or ethyl methyl carbonate (EMC). The performance of lithium-ion batteries is dependent on the solvent dielectric constant as well as viscosity.
In this application note, we probe the viscosity of battery solutions with varying ratios of DMC, EMC, and EC as a function of temperature. In addition, we calculate the activation energy as a function of EC to DMC & EMC ratio from Arrhenius fits to the viscosity vs. temperature results.
Fill out the short form to the right to download the full Application Note!

.jpeg?width=300&name=Woman%20scientist%2c%20documents%20and%20hands%20writing%20_575258496-min(1).jpeg)
TOUCH
Read
Applications
ReadApplications

Read
Testimonials
ReadTestimonialsGo to page

Browse
Webinars
Browse WebinarsGo to page
.png?width=200&height=58&name=RheoSense%20Logo%20(REGISTERED).png)